The VAGUS Nerve (Part II): What Happens When It Goes Bad? Vagally-Mediated Voice Symptoms and Neurogenic Pain Syndromes
At-A-Glance
- This is the second installment of a three-part article on the vagus nerve; this one covers phonatory (voice) symptoms and neurogenic pain syndromes … there is a lot of new medical information here. If you haven’t already done so, I recommend that you first read The Vagus: Part I.
- Vagal neuropathies (“sick-nerve syndromes”) can affect the voice; and symptoms include voice change, vocal fatigue, effortful phonation, loss of singing power and pitch-range, and odynophonia (voice-use pain). In addition, vagal neuropathies can cause nerve pain in the throat, chest, and back.
- Next week, The Vagus Part III will cover gastrointestinal, respiratory, and cardiovascular sequelae and symptoms of vagal neuropathies.
Dear Reader: Until now, there has been a paucity of information about the vagus nerve and vagally-mediated neuropathic syndromes. This topic is impactful and this blog is expansive; therefore, I have divided it into three parts. This is Part II, but the three parts taken together are my “White Paper on the Vagus Nerve”… rather like a medical tell-all from an experienced physician. Prego! Most of what I know is here, including clinical observations that have not been previously published. -Dr. Jamie Koufman
Before I recognized respiratory reflux for what it was, I was just a laryngologist, a physician specializing in disorders of the voice, vocal cords, and throat … and I still am. But with one unforgettable case in 1981, I discovered laryngopharyngeal reflux (LPR).†
†footnote: Because my patients didn’t have GERD, I coined the term LPR (1987). Within a few years, I had proved and reported LPR … over the next three decades, my research, especially basic science work with Dr. Nikki Johnston flourished. If you are interested in the arc of my career, here are my Curriculum vitae; lectures begin on page 6, book chapters page 35, and peer-reviewed publications begin page 39.
When I went into practice in 1978, I had state-of-the-art instrumentation for examining the larynx and throat, transnasal flexible laryngoscopy (TFL). It was performed with an ultra-thin endoscope with a high-resolution camera, slid into the patient’s nose painlessly, to examine the voice box and throat. In my first few years in practice, in addition to discovering LPR, I also observed that many voice disorder patients had weak, asymmetric, and/or bowed vocal cords. As it turns out, they had vocal cord paresis (partial paralysis); and notably, many of those patients reported voice symptoms beginning after an upper respiratory infection (URI).
For more information about the vagus nerve and many types of vagus nerve problems — and why most physicians are so generally ignorant about this topic — I refer you to a lecture that I gave at Boston University Medical School the June before Covid, The New Field of Integrated Aerodigestive Medicine.
PHONATORY (VOICE) SEQUELAE OF VAGAL NEROPATHIES
By far the most common vagal neuropathy is vocal cord paresis (VCP), and frequently it is bilateral (both sided). That isn’t to say that some patients don’t present with complete vocal cord paralysis; but in my experience, the ratio of partial- to complete-paralysis patients is about 20:1.
How do I know that VCP is vagal? In addition to TFL showing findings of VCP, since 1987 I have been performing diagnostic laryngeal electromyography (LEMG), which measures “electrical” vocal cord (vagal nerve) function. LEMG is the diagnostic sine qua non for vagal neuropathy; LEMG alone proves a neuropathy exists.
How To Diagnose Vagal Neuropathy and Vocal Cord Paresis?
For diagnosis of vocal cord paresis (VCP), i.e., vagal neuropathy, there are three criteria: symptoms, laryngeal findings, and laryngeal electromyography (LEMG).
Symptoms: The first diagnostic criterion for VCP is the “glottal closure index.” There are four symptoms common in patients with vagally-mediated VCP. For all patients with voice problems, I have them score each of the four items/symptoms from 0-to-5, with five being most severe; see below. Then, the scores for each of the four items are totaled for the Glottal Closure Index (GCI), a useful diagnostic measure in clinical practice.
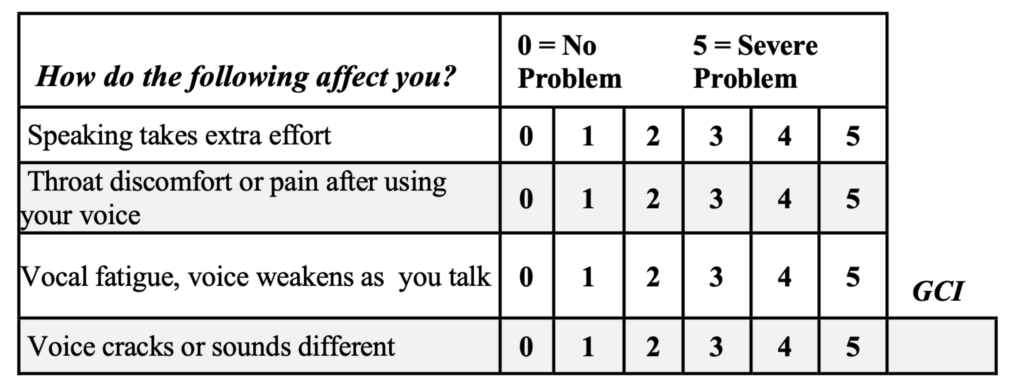
A GCI score of 10 or more is usually associated with VCP; and for patients whose GCIs are in the 16-20 range, you can bank on paresis.
For singers and other vocal professionals, there may be additional unfortunate paresis symptoms, namely, loss of voice strength and pitch-range. Indeed, for high-level singers like opera singers, VCP can be career ending. Thankfully, this happens very infrequently.
Of the VCP symptoms, voice-use pain is the most certainly neurogenic; I coined a new term for this, odynophonia, painful phonation. This symptom can be disabling; however, medical treatment is usually successful, but more about that in a minute.
Are GCI voice symptoms really neurogenic? VCP symptoms appear to be neurogenic for three reasons. First, most people with VCP can date the onset of their symptoms to a precipitating illness or event. Second, people with bad voices for other reasons (not paresis) may have vocal fatigue, but they almost never have the other voice symptoms, especially odynophonia. Finally, symptomatic VCP patients usually respond beautifully to medications used to treat other neurogenic conditions.
Examination: The second criterion for VCP is having the right laryngeal findings on TFL with videostroboscopy, which is a technology that allows the examiner asses vocal cord vibrations. The most common findings of VCP on TFL are: (1) A sluggish or hypomobile vocal cord, (2) Vocal cord bowing in a patient under 60 years of age, (3) Inability to close the vocal cords without increased effort, (4) Asymmetric vibrations between the two vocal cords on stroboscopy.
Note: For those performing TFL, I use the repeated “eee”/sniff/“eee”/sniff maneuver to help bring out paresis findings.
LEMG: The third criterion is laryngeal electromyography (LEMG) that unequivocally shows neuropathy. Using a needle electrode, I examine the electrical activity in four muscles of the larynx to test four vagus nerve branches, the right and left recurrent laryngeal- and superior laryngeal nerves. This test is the diagnostic sine qua non for vagally-mediated vocal cord paresis and paralysis. LEMG provides the proof of neuropathy that no other test can provide.
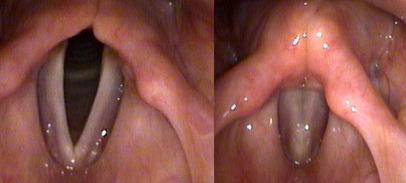
Normal: This is a normal larynx on TFL with the vocal cords open during breathing (left) and “closed,” vocal cords touching in the midline along their lengths, during phonation (right). Notice that the vocal cords come together like two hands clapping on a hinge, with the hinge being at the bottom. FYI: When viewing vocal cords by TFL like this, the right vocal cord is on the left, and the left vocal cord is on the right.
Case Illustration: A 47-year-old man had the flu, and three days later, he lost his voice completely Examination showed paralysis of the left vocal cord as well as a weakened, partially paralyzed, right vocal cord. Three days after onset, he began coughing and had LPR symptoms. Did the virus knock out his vagus nerves?
Vagal neuropathies? You bet, both sides. The nine-frame, action sequence (below) is taken from this patient’s TFL exam.
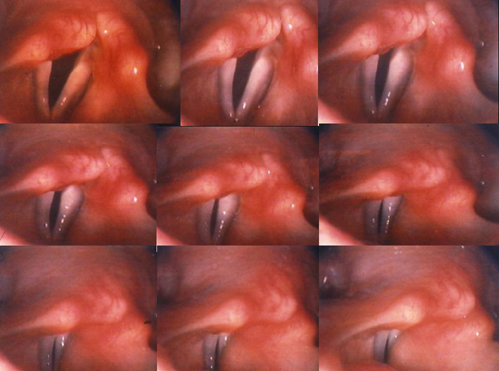
On phonation, his vocal cords never close, never touch, like the normal example. This sequence of nine photos (from top left to bottom right) was taken when the patient was asked to phonate, say “eee.” In the nine consecutive frames the vocal folds try to get together, but never actually touch. In the bottom right image, you can see that there is an opening between the two vocal cords. Though relatively severe, this is what bilateral vocal cord paresis can look like, again, nothing like the closed vocal cords seen on the right photo in the normal example above.
In 2001, I coined the term Post-Viral Vagal Neuropathy (PVVN) to describe a clinical entity that I saw often: Viral URIs leading to damage of the vagus nerve or nerves, precipitating and resulting in a host of syndromes and symptoms.
The symptoms don’t necessarily appear immediately; sometimes they begin days-to-weeks after the URI (e.g., cold, flu, sinusitis, bronchitis). Interestingly, in my experience, Covid is an uncommon cause of vagal neuropathy; however, it does often exacerbate reflux in people who had reflux before Covid. (BTW, I just had a case that I believe was Covid causing vagal neuropathy; but this appears to be uncommon.)
With PVVN, the typical LEMG shows involvement of all four laryngeal nerves with decreased recruitment and polyphasic motor units. Even if the patient does not have a history of a clear-cut precipitating URI, the pattern on LEMG may be similar to PVVN. I believe that PVVN may be “silent.” This is similar to another viral neuropathy, Bell’s palsy (“idiopathic” facial paralysis). About half of Bell’s patients there is a preceding URI, and for the other half, not.
By LEMG, when there are very few motor units seen, the prognosis for spontaneous recovery of the nerve is not great. On the other hand, when there are plentiful low-amplitude polyphasic (so-called “nascent”) motor units, the prognosis for recovery is good; although full recovery may take up to two years.
LEMG alone provides the diagnosis and prognosis of vagal neuropathies. Unfortunately, there are very few laryngologists performing diagnostic LEMG because the equipment is expensive, it takes training to perform properly, it is uncomfortable for the patient, and the insurance reimbursement for LEMG is low.
To reiterate, symptomatic VCP (e.g., voice change, loss of voice volume and pitch-range, vocal fatigue, effortful speaking, voice-use pain) is the most common vagally-mediated neurogenic syndrome in clinical practice.
How Common Are Vagal Neuropathies, VCP and PVVN?
How common are vagal neuropathies in the population? What is the prevalence of VCP? Here are my data from a “normative” study done thirty-five years ago, but never published.
I studied the LEMGs of a group of “normals” who weren’t exactly normal. At the time, I was treating a large group of patients with spasmodic dysphonia (SD), which is an isolated dystonia, a central (brain) movement disorder, which renders the voice herky-jerky and almost unintelligible. SD can be disabling. The good news is that SD treatment with small doses of Botox injected into the vocal cords restores most SD-sufferers voices to normal or near-normal for a few months. The treatment does need to be repeated a variable amount of time depending on severity of the SD and the vocal needs of the patient.
So, when a new SD patient came to me for Botox, never having had it before, a “Botox virgin”; before I gave the first Botox injection, I would perform a diagnostic LEMG. This made good sense since VCP might change the dose of Botox and/or voice outcome. (Smaller doses for patients with VCP.) And, diagnostic LEMGs could only be done in “Botox virgins,” since after Botox, subsequent LEMGs have little value, because Botox itself creates the electrical appearance of VCP.
My “normals” had a central (brain) neurological movement disorder, so I assumed that no peripheral (vagal) neuropathy would be expected with a SD diagnosis. Thus, I had a more-or-less normal population to study: the LEMG data showed that 40% (17/43) of the study group had VCP.
Though the sample size is small, I think that it is fair to estimate (in the ballpark) that approximately 40% of the general population has VCP; and presumably most are asymptomatic. That seems a big number; but remember, the vagi lie just under the lining membranes of the throat, and presumably it’s there that they get infected or damaged over a lifetime in a lot of folks..
If 40% of the population with VCP seems an unexpectedly big number, but consider this, 80-90% of my patients with voice disorders have VCP … I have not formally analyzed the data, but 80-90% is close to the real number based upon laryngeal examinations and the LEMG data from hundreds of patients.
I have clinical and research experience with thousands of patients with vagal neuropathies; and much of the material here has not been reported in peer-reviewed medical journals. I would do so, but in semi-retirement, I lack the resources to do so. Besides, I often have difficulty getting my work published in “prestigious” medical journals, because the ideas (like the ones in this article) are considered too controversial, even radical. That said …

I believe that vast majority of voice disorder patients have LEMG-documented VCP and/or pH-documented LPR, usually both; and this paradigm-shifting postulate is contrary to the opinions of most of my medical colleagues. That said, I call those who don’t get the new paradigm “Flat-ists” … well, and guess what my friends … the world is round!
Furthermore, reflux and paresis are the right pre-morbid conditions — inflammation and altered (compensatory) laryngeal biomechanics — for the development of vocal cord growths, e.g., vocal cord nodules, polyps, granulomas, leukoplakia (white plaque), and cancer. VCP also causes vocal cord bowing, thinning, and atrophy.
By the way, voice misuse and abuse syndromes alone are rarely the cause of those lesions. Furthermore, the prescription of long-term voice therapy does not help these VCP patients very much, and I stopped recommending it routinely. That said, I do think patients who demonstrate maladaptive compensatory behaviors should see a speech-language pathologist trained in voice therapy for a short period of time, perhaps a few weeks. But voice therapy should be abandoned when voice improvement plateaus.
This next illustrative case will hopefully bring my paresis paradigm together.
Illustrative Case Example: Diagnosis and Treatment of VCP Secondary to PVVN
A well-known, previously-healthy, 40-year-old actress presented with severe voice symptoms three weeks after having had an upper respiratory illness. Her voice was breathy, raspy, strained … and quite obviously terrible. She also complained of vocal fatigue, effortful phonation, and debilitating voice pain. Also, she had respiratory reflux (LPR) symptoms: too-much throat mucus, chronic throat-clearing, globus, and coughing when she lay down.
Another doctor had put her on voice rest with no benefit. The patient was frantic because she was due to start filming a movie in California in three weeks time, and she knew that she would have to yell and scream in her role. She came to see me knowing that she was in trouble.
Diagnosis: Her Examinations:
(1) Videostroboscopy (TFL laryngeal exam) revealed moderate nocturnal reflux and bilateral vocal cord paresis with a tiny gap between the vocal cords; in addition, there were compensatory muscle-tension dysphonia biomechanics, and on stroboscopy, stiffness and mucosal wave asymmetry. (2) Laryngeal electromyography (LEMG) was abnormal; from report, “LEMG examination was consistent with a neuropathy affecting both superior and recurrent laryngeal nerves; the pattern is consistent with a relatively-recent post-viral vagal neuropathy. In addition, there is evidence of on-going neural regeneration as evidenced by the presence of low-amplitude, polyphasic (so-called “nascent”) motor units. The prognosis for recovery is relatively good, and no further neurological work-up is needed.”
The diagnosis of PVVN was confirmed. But remember, she was scheduled to start shooting a film in a few weeks.
Treatment: I started her on a strict antireflux program; and in this case, because of “maladaptive laryngeal biomechanics,” I did refer her to a speech-language pathologist, “voice therapist,” for some therapy. I also started her on medications for neurogenic symptoms: amitriptyline 10 mg. before bed and gabapentin 100 mg. four times a day (before meals and at bedtime). I stayed in close contact with the patient, and after two days, I increased the gabapentin to 200 mg. x 4 per day (total dose 800 mg.); four days after that, I increased the gabapentin to 300 mg. x 4 per day (total dose 1,200mg.)
The patient was tolerating the medication with no side-effects, but her symptoms were only marginally improved, so I continued to escalate the gabapentin every four days: from 400 mg. x 4 per day (total dose 1,600 mg.) to 600 mg. x 4 per day (total dose 2,400 mg.). At that point, twenty-days into treatment, she reported great relief.
Her voice was almost normal and she no longer had neurogenic voice symptoms, She was still on her antireflux program and with the help of the neurogenic medications, she went to her California to shoot her film … confident that she could do it.
Six months later, she was back in New York. By that time, we had tapered her gabapentin to one dose at bedtime; and six months after that, she was off all meds and still symptom free. On examination at that time, she still had some paresis findings with much-improved laryngeal biomechanics, and her reflux was under good control.
Caveat: Medical Treatment? When and How Much?
The above case is unusual; in the parable of the tortoise and the hare, this is the hare. In other words, it is not often that I would escalate gabapentin to such a high dose so quickly. FYI: Although, there are few side-effects of gabapentin, if one does increase dose quickly as in this case, there may be some problems (temporarily) with word retrieval.
Now the tortoise: The details of how I usually start treatment with amitriptyline and gabapentin in a conservative and stepwise fashion is provided in my Neurogenic Cough Blog. Go there before considering asking your doctor for any prescriptions. There is right way to escalate these medications and with every dose change, you, the patient must ask if your symptoms are better, worse, or the same, and if you are experiencing any side effects. If doses start small and are increased incrementally and gradually, side effects should virtually never occur.
It is also important to re-emphasize that most patients with VCP don’t need “neurogenic” medical treatment, especially those with reflux as a cofactor. Fix the reflux first, and most people with mild paresis will get well whether or not the VCP is treated.
So, the purpose of this post is to call attention to vocal fold paresis, post-viral-vagal neuropathy, and a new treatment algorithm for those neuropathic conditions. Treatment works well for most patients, and so I believe that the medical community should take notice — vagal neuropathies are common..
NEUROLOGICAL: VAGAL NEUROGENIC PAIN SYNDROMES
I am almost hesitant to write this section, lest many people read it, and then silently mutter, “This is me”; vagal neuropathies cannot be blamed for everything.
I have already covered odynophonia; but that said, throat pain, that is, chronic sore throat, especially burning throat, is not a typical reflux symptom; it’s neurogenic. However, that said, reflux certainly can make this type of neurogenic pain worse.
Chronic throat pain is usually neurogenic and the diagnosis can be confirmed by LEMG, and in my opinion, successful medical treatment confirms the diagnosis. The medical treatment for chronic sore throat is similar to that for odynophonia (voice use pain) covered above.
Illustrative Case Example: I had a young woman from Kansas come see me with debilitating and disabling throat pain. Previously, she had had three unsuccessful surgeries: a tonsillectomy, removal of her lingual thyroid, and removal of parts of her hyoid bone. The latter was done for a diagnosis of Eagle’s Syndrome, in my mind, a very questionable diagnosis. Actually, I think that “Eagle’s syndrome” is NOT a thing; severe throat pain is almost always neurogenic.
I started her on low dose amitriptyline and gabapentin, and over the course of six months escalated her doses of gabapentin. I recall that she came back for reevaluation about a year later (in July): I found nothing new. But WE were still convinced that she had neurogenic pain, and so I sent her home on gabapentin 800 mg. x 5 per day (total dose 4,000 mg.). (We had gradually escalated to that very high dose.)
The following October, she was back in New York, happy as could be — she no longer had any pain! But the 4,000 mg. of gabapentin that I prescribed did not give her relief; but since she was sure that she had neurogenic pain, she kept asking her local doctor to increase the doses of gabapentin in 800 mg. increments. My goodness! She was then taking 8,000 mg. with complete relief of her pain.
She remained on treatment for two years, and subsequently was tapered to 800 mg. of gabapentin at bedtime. I don’t know, but she still may be taking that, now, many years later.
Treatment of Vagally-Mediated Neurogenic Symptoms
The above case represents the highest dose of gabapentin that I have ever seen, but it worked for the patient with no untoward events or symptoms … and there is a message in that. (BTW, since that patient, I have used high-dose, 4,000 mg. per day, in a handful of patients.)
Gabapentin comes in 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg. doses; that’s a huge spread. That they manufacturer from 100 mg. to 800 mg. pills should tell you that people have quite varied dose responses to this medication, and that the effective-dose range must vary very widely, which it does.
For patients who complain of burning throat, most have both VCP and reflux; and I treat both simultaneously. Above, we have a small woman taking 8,000 mg. a day, but at the same time I have a huge man with neurogenic cough who responded very well to gabapentin 100 mg. twice a day. Again, there is a big dose range for successful treatment of neurogenic symptoms.
Who do I treat? The huge confounding cofactor in this discussion about neurogenic symptoms is respiratory reflux (LPR). If a patient has reflux and a neurogenic symptom, I usually treat the reflux first. That’s because neurogenic symptoms are usually made much worse by reflux. Fix the reflux and the neurogenic symptoms may abate. But if neurogenic symptoms are disabling, I treat them along side of the antireflux treatment.
Who I treat depends on the severity of the symptoms, my certainty that the problem is neurogenic, and the needs of the patient. For severe odynophonia (voice-use pain) in a vocal professional, say a Broadway singer with eight shows a week, I am aggressive in escalating gabapentin doses, as in the actress case above. I also tend to treat new cases of PVVN as I think that there is no reason to take a wait-and-see attitude in such symptomatic cases.
Treatment Side Bar: Amitriptyline and gabapentin seem to work together better that either does separately. Some (few) patients do well an amitriptyline alone, but more often than not, I recommend a small dose of amitriptyline while slowly titrating the gabapentin up to an effective dose.
Illustrative Case Example: A 56-year-old woman consulted me with a ten year history of continual, severe, dry, burning throat. She thought it was worse when she had reflux, but her only respiratory reflux symptoms were post-nasal drip and too-much throat mucus. Interestingly, she remembered having an URI prior to onset of the throat pain a decade before. She was on already on a reasonable antireflux program before doing the virtual consultation with me. I told her that in my opinion her symptoms were neurogenic, and I recommended that she go on amitriptyline and gabapentin, starting slowly as outlined here; and I left her to discuss this treatment with her primary care physician.
Throat Pain: A Restrain Refrain: Throat pain that congers up a diagnosis of “Eagle’s syndrome” in the clinician’s mind should be assumed to be neurogenic pain, and the patient should be spared unnecessary throat surgery.
How long do I treat? I leave patients who have relief of neurogenic cough on treatment at least one full year, after the cough is gone, not just improved, but gone. That’s because if the medicine is stopped too soon, recurrence is more likely. I like to think that over time (many months), there is a reset in the vagal nucleus. I don’t know if this is true but neurogenic coughers in my practice stay on the effective dose a minimum of a year. I tend to treat patients with disabling throat pain similarly; however, for “lesser” symptoms, I may taper earlier.
How do I taper? When the patient has been asymptomatic for months-to-years, tapering can begin. I recommend dropping one dose per month, starting with (omitting) the morning dose of gabapentin (regardless of milligrams). Then, an additional dose can be dropped each subsequent month. The order of discontinuance that I recommend is breakfast, then lunch, then dinner, then bedtime doses.
The slow tapering is used to test if any of the neurogenic symptoms return. By the way, if the patient is taking amitriptyline, too, that is discontinued last. Again, I believe that small doses of amitriptyline augment the beneficial effects of the gabapentin, so I tend to use them together, but only10 mg. of amitriptyline at night.
Clearly, this is not an exact science, and executing this type of treatment should be reserved for physicians who understand neurogenic conditions and who are able to maintain close contact with this type of patient.
Uncommon Vagally-Mediated Neurogenic Pain Syndromes
What is the incidence of each neuropathic syndrome in my practice? The most common is odynophonia associated with vocal cord paresis, next in frequency comes neurogenic cough (covered before), Much less common, but next in frequency, is throat pain. (Should there be a new term medical for neurogenic throat pain, “odynopharyngis,” perhaps?) Finally, the least common vagally-mediated neurogenic conditions are atypical back and/or chest pain; in my experience, those make up less than 1% of vagally-mediated neurogenic syndromes/symptoms.
Some people have epigastric pain that goes through to the back, usually to the shoulder or the shoulder-blade area (either side, but not both). If this occurs after meals and in the evening, it is probably “atypical heartburn.” If reflux-related, then reflux treatment is helpful; however, there are some patients who have this type of pain all day long without necessarily having reflux; these cases are usually vagally-mediated and neurogenic.
Of the chest pain variants, the most common neurogenic symptom is “esophagus burn.” These patients point at their sternum and say that the burning is there all the time. Note: this burning pain is different than heartburn, and the persistence of it puts the vagus and vagal neuropathy in the crosshairs. Needless-to-say, all patients with chest pain and referred shoulder pain should be seen by a physician to rule out serious conditions, e.g., cardiovascular or neoplastic disease (cancer).
Honorable Mention Goes to Burning Tongue
Not Vagal But … When I was a resident, we were learned that the patient you did not want to see was an old lady with a burning tongue. That was because the patient was miserable and wanted relief, but we usually didn’t know what caused it, let alone how to treat it. So, after ruling out B12, zinc, iron, and folate deficiencies, that can sometimes cause burning tongue, by blood test … the clinician was/is left with the possibility that burning tongue might be neurogenic. This post is about the vagus nerves, but in the case of burning tongue, the culprit is the hypoglossal nerve (CNXII) or possibly even the facial nerve (CVVII). So, as it turns out, burning tongue often can be remedied with relatively low-dose amitriptyline and gabapentin, again see the Neurogenic Cough Blog for this information.
Closing Shot Across the Bow
To this day, most otolaryngologists (ENT doctors), including specially-trained laryngologists, still massively under-diagnose both VCP and respiratory reflux (LPR); and alas, as a consequence, their patients receive inappropriate and/or suboptimal care.
References
Koufman J, Wiener GJ, Wu WC, Castell DO. Reflux laryngitis and its sequelae: The diagnostic role of 24-hour pH monitoring. J Voice 2:78-89, 1988.
Koufman J. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD). Laryngoscope 101 (Suppl. 53):1-78, 1991.
Amin MR, Koufman J. Vagal neuropathy after upper respiratory infection: A viral etiology? Am J Otolaryngol 22:251-256, 2001.
Koufman J, Walker FO, Joharji GM. The cricothyroid muscle does not influence vocal fold position in laryngeal paralysis. Laryngoscope 105:368-372, 1995.
Koufman J, Postma GN, Whang C, et al. Diagnostic Laryngeal Electromyography. Otolaryngol Head Neck Surg 124:603-606, 2001.
Koufman, JA, Postma, GN, Cummins, MM, Blalock, PD. Vocal fold paresis. Otolaryngol Head Neck Surg 122:537-541, 2000.
Bach KK, Belafsky PC, Wasylik K, Postma GN, Koufman J. Validity and reliability of the glottal function index. Arch Otolaryngol Head Neck Surg 131:961-4, 2005.